Looking beyond the horizons of today’s obesity therapeutics with the goal of achieving healthier, sustainable outcomes.
Obesity therapeutics: Unmet needs
Obesity Pipeline Overview
Number of Assets by Phase
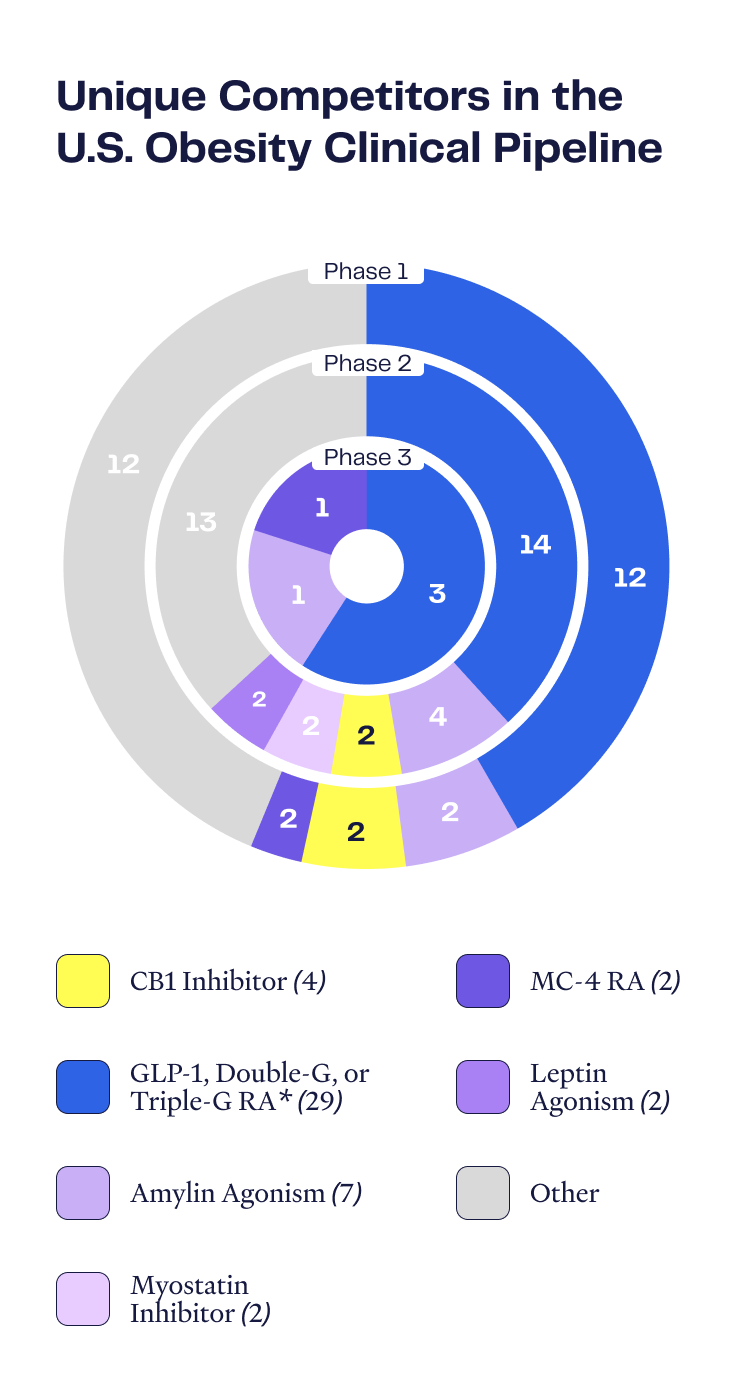
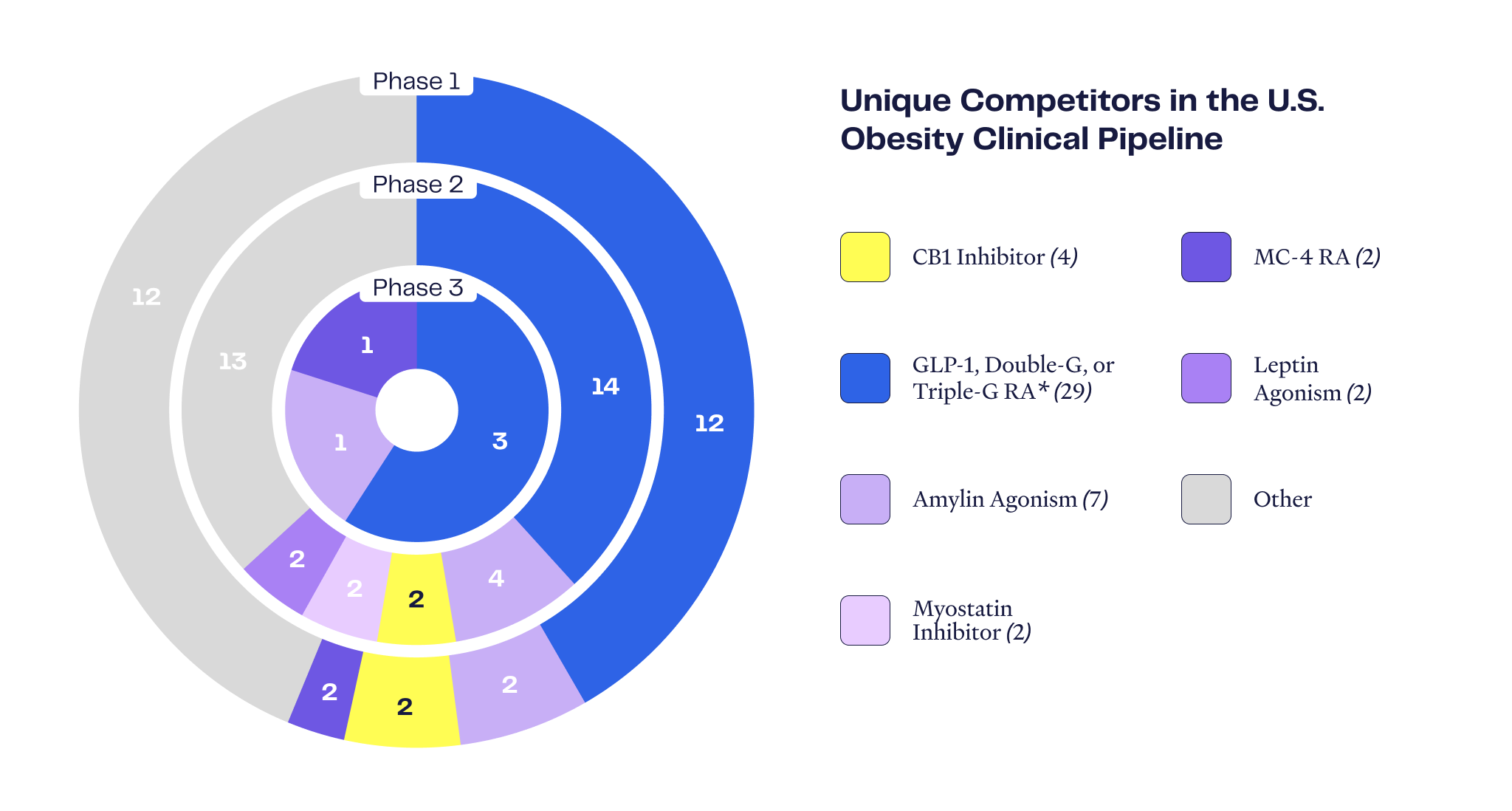
Certain “mature” disease areas have many different commercialized therapeutic drug choices to meet myriad patient requirements, yet the obesity space is at a relatively early stage of development and served by a narrow set of therapeutic options. There are only seven FDA-approved anti-obesity drugs, and commercialized and Phase 3-stage drugs are dominated by incretin-focused mechanisms (GLP-1 and GIP). There is significant opportunity for new drugs with mechanisms of action that may avoid the shortcomings of the incretin drugs.
Peripheral CB1 inhibition: a mechanism with positive evidence and promise
Complementary, Not Competitive
CB1 impacts key metabolic pathways that complement existing products & strategies
Considering the side effect profile of the incretin drugs and the capabilities and limitations of other anti-obesity mechanisms of action, CB1 inhibition offers attractive attributes to potentially play an important role in serving the growing and diverse needs of patients with obesity and overweight.
Nimacimab: a differentiated peripheral CB1 inhibitor
Non-Incretin Product Landscape
Assessing nimacimab’s utility in the CBeyondTM Phase 2 study
The results of this study will help guide Skye’s development activities within the heterogeneous obesity market.
- Van Gaal et al., Efficacy and Safety of Rimonabant for Improvement of Multiple Cardiometabolic Risk Factors in Overweight/Obese Patients. Diabetes Care, Vol. 31, SUPPLEMENT 2, FEB 2008
- Wilding et al., Once-Weekly Semaglutide in Adults with Overweight or Obesity. New Eng J Med, 384;11, 2021
